On 13TH June PlumeStars participated at Place Conference 2024 located in Rome: the Place Conference is a platform of laboratories for advance in Cardiac Experience.
Read more “PLUMESTARS ATTENDED THE PLACE CONFERENCE IN ROME”

On 13TH June PlumeStars participated at Place Conference 2024 located in Rome: the Place Conference is a platform of laboratories for advance in Cardiac Experience.
Read more “PLUMESTARS ATTENDED THE PLACE CONFERENCE IN ROME”

February 29th is the World Day of rare diseases. In Italy there are around 2 million people suffering from this pathology. A rare disease is a pathology that affects limited number of people, precisely 1 out of 2,000. and for this reason, is difficult to identify and to treat. This means that many subjects don’t have a certain diagnosis and cure. The rare disease also directly affects the families and friends of the affected that need constant assistance and support.

PlumeStars participated at AAPS 2023, an annual Conference of the American Association of Pharmaceutical Scientists, held on 22nd, 23rd, 24th and 25th October at Orange County Convention Center, Orlando (Florida).
This important event featured be keynotes, demonstrations and grand presentations, with insights for both investors and innovation-driven organizations.
Read more “PLUMESTARS PARTICIPATED AT THE AAPS 2023 EXHIBITHION”

PlumeStars participated at the 62nd AFI Symposium, the Italian Conference of the Industrial Pharmacists, held on 7th, 8th and 9th June at the new Pala Congressi in Rimini.
From 31/05/2023 to 01/06/2023, Prof. Paolo Colombo invited by the University of Liege (B), as Emeritus of University of Parma and CEO of PlumeStars, entered the nominated Committee for the evaluation Audit of the Center of Interdisciplinary Research on Medicines (CIRM)
The other members of the committee were:
Read more “Prof. Paolo Colombo invited by the University of Liege”
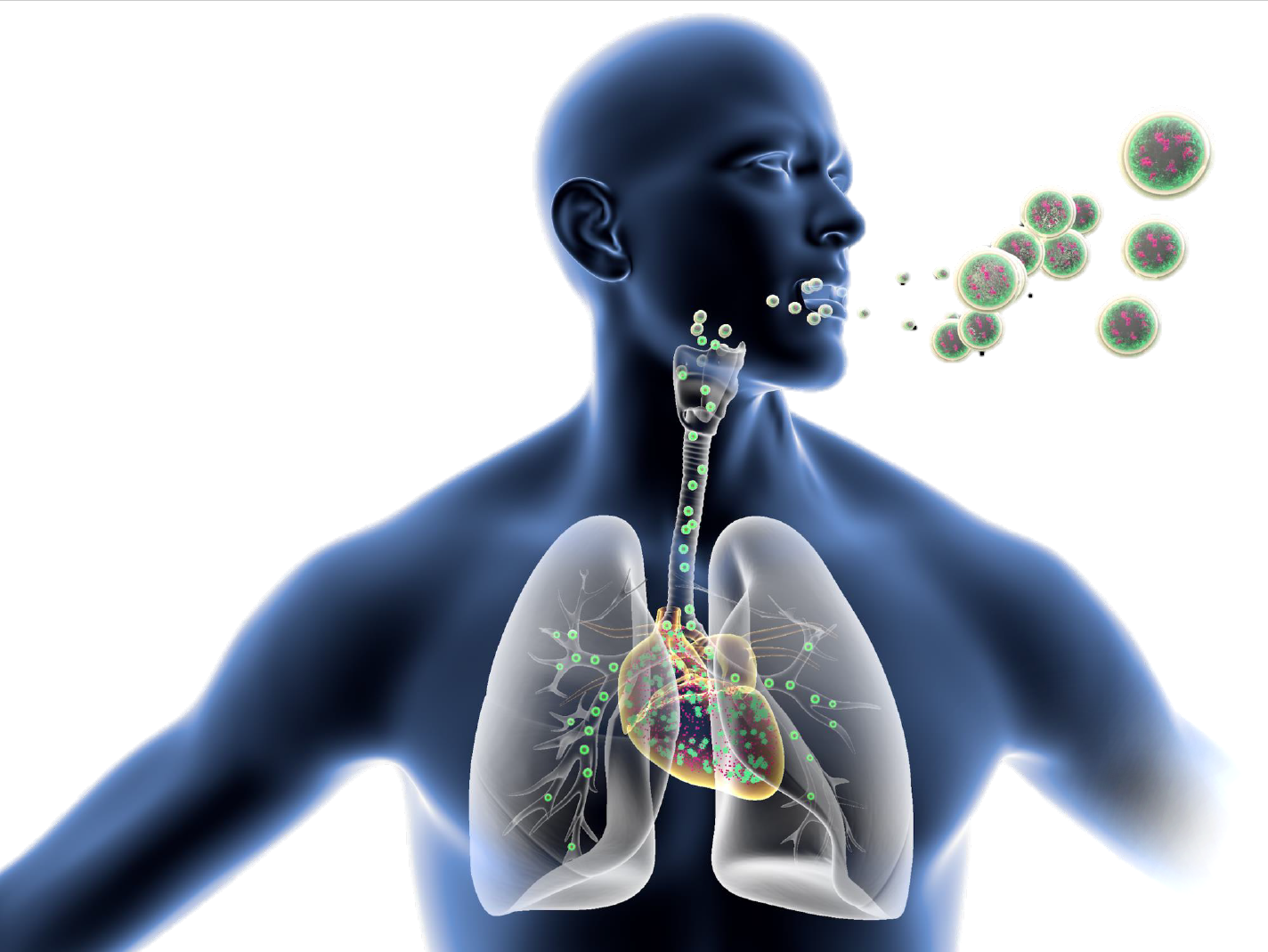
Prof. Paolo Colombo, CEO of PlumeStars srl, has been invited as speaker at National and Kapodistrian University of Athens on 18th January to present the speech “Nanoparticles lung inhalation as a drug delivery route for heart diseases” to the participants of the annual meeting of the MSc Industrial Pharmacy, a post graduate course directed by Prof. Dimitrios Rekkas. The meeting was very well attended by several representatives of the Greek pharmaceutical companies.
Read more “PROF. PAOLO COLOMBO SPEAKER AT UNIVERSITY OF ATHENS”

On June 8th PlumeStars srl participated at the “Venture Contest for International Entrepreneurs -Win in Suzhou Win the Future”, an international competition for innovation and entrepreneurship of new projects and talents, borned in Suzhou (China) since 2011.

After the Covid 19 Pandemic, trade fairs restart including the AFI Symposium. Also for this year, PlumeStars participated at the 61st AFI Symposium, the Italian Conference of the Industrial Pharmacists, held on 8th, 9 th and 10 th June at the new Pala Congressi in Rimini.

PlumeStars adhered to the “Manifesto for Eu covid-19 research” proposed to reaffirm the key role of Research & Innovation for the future of Europe and its citizens.
Read more “PlumeStars joined at “Manifesto for Eu covid-19 research””
Also for 2022, the Lifebility of Lions charity is proposing the Lifebility Award. The competition years has been aiming to find highly innovative ideas and projects that can help solve problems in our society. The watchword has always been “ethics”, a fundamental requirement for proposing projects that have an eye on the present and the future, and on the critical issues that hinder our society.
Read more “PlumeStars participates at Lifebility Award 2022”